First, Some History
2000 years ago, the area now called India, originally Bharat, had artisans who knew how to make steel that did not rust. Science was lost when the Mongols invaded and burned the Library around 1306.
Then, Some Science
Around 2003, the famous professor from the Indian Institute of Technology, R. Balasubramanian tested the Iron Pillar of Delhi, one artifact of iron from the early period that has been rust-free for 1600 years. He learned that the cause of the extreme corrosion resistance was the formation of an amorphous layer of FeOOH. FeOOH is the most stable form of iron because it cannot oxidize further. Most forms of FeOOH are crystalline and flake-off. But an amorphous layer is impermeable and stays put. This amorphous layer of FeOOH is called Misawite – named after the famous Japanese scientist who predicted in 1970 that an amorphous layer of FeOOH could form – though it had not been seen in nature at that time.
The EonCoat Revelation
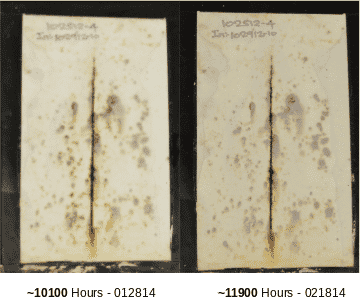
In 2013, we applied EonCoat to a rusted panel and placed it in ASTM B117 (the salt fog chamber). At around 4000 hours the coating began to show dark spots and we thought it would soon fail. But it did not fail and began to look better. Around 7500 hours the panel showed many dark spots and we believed massive failure was imminent. But again, the panel returned to its original look, and by the 11,900 hours mark the coating looked much better than it did at the 7500-hour mark. It seemed to have healed itself.
The EonCoat Science
Our scientists wanted to understand what could possibly cause a panel to look like it was heading for massive corrosion and then suddenly heal. We preserved half of the panel for posterity and the scientists dissected the other half for extensive analytical study. We used scanning electron microscopy, EDS, Raman spectroscopy, and a host of other tools to help us understand the behavior. It was the Raman Spectroscopy that ultimately answered the question. We found that the rust had been catalyzed by the phosphate in EonCoat and had turned to Misawite.

EonCoat Science & Iron Pillar Science Meet
From our history, and from Misawa, we know that once Misawite forms the corrosion process effectively stops. If EonCoat’s chemistry serves to convert rust into Misawite it means that the steel will remain protected for an extremely long time. Many of the iron artifacts from the early period in Indian history have remained rust-free for over a thousand years. It looks like we have discovered a way to duplicate what Indian artisans knew how to do 2000 years ago. How is that for progress?

Ready to Learn More About EonCoat?