Watch this video or continue below to read the full transcript of the video.
Finding a Protective Layer
EonCoat forms an iron phosphate alloy layer that chemically bonds to steel to prevent corrosion.
Let’s take a closer look at how that process works.
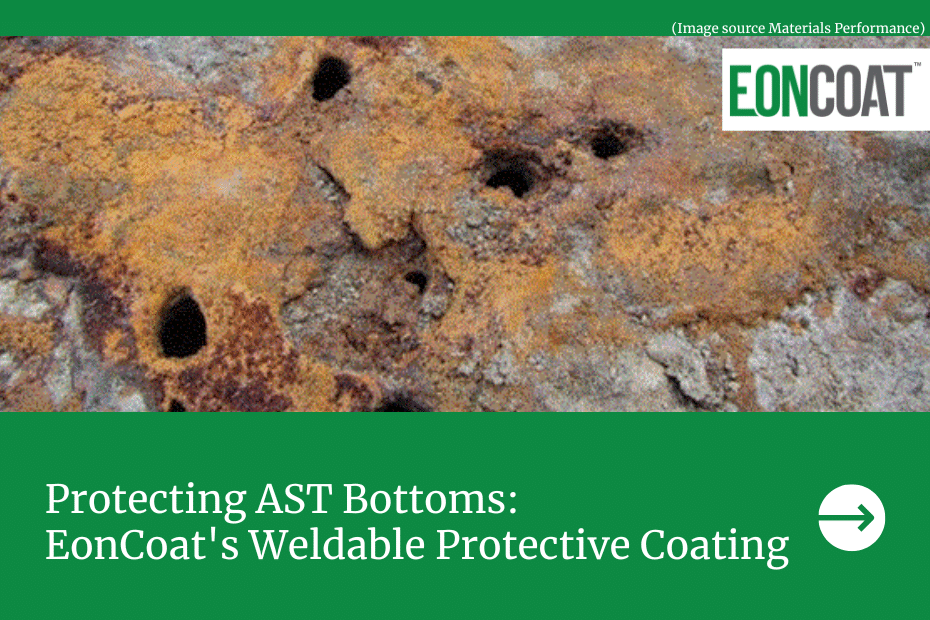
Steel rusts because individual steel atoms lose electrons as they transmit electrical current. To balance their electrical charge, steel atoms bond with oxygen, creating rust. But if steel atoms are given something else to bond with, like phosphate or silicate, a protective layer forms on the steel instead of rust.
The Problem with Primers
Knowing this, paint companies have added phosphate and silicate to their primers. But the phosphate in paint is encapsulated in a polymer that functions like glue and doesn’t let the phosphate out quickly enough. And there is no way to load enough phosphate into the paint to satisfy all the positively charged iron atoms.
EonCoat Offers the Solution
EonCoat solves these problems by chemically bonding the phosphates and silicates directly to the metal, rather than trapping them in a gluey resin. The result is that EonCoat’s phosphate volume is dozens of times greater than the phosphate-silicate load in polymer coatings.
Best of all, the bonding mechanism in EonCoat that holds the inhibitors to the steel is the iron phosphate alloy layer, and that alloy layer only gets thicker with time.
Allow EonCoat to protect your steel from corrosion.

Ready to Learn More About EonCoat?