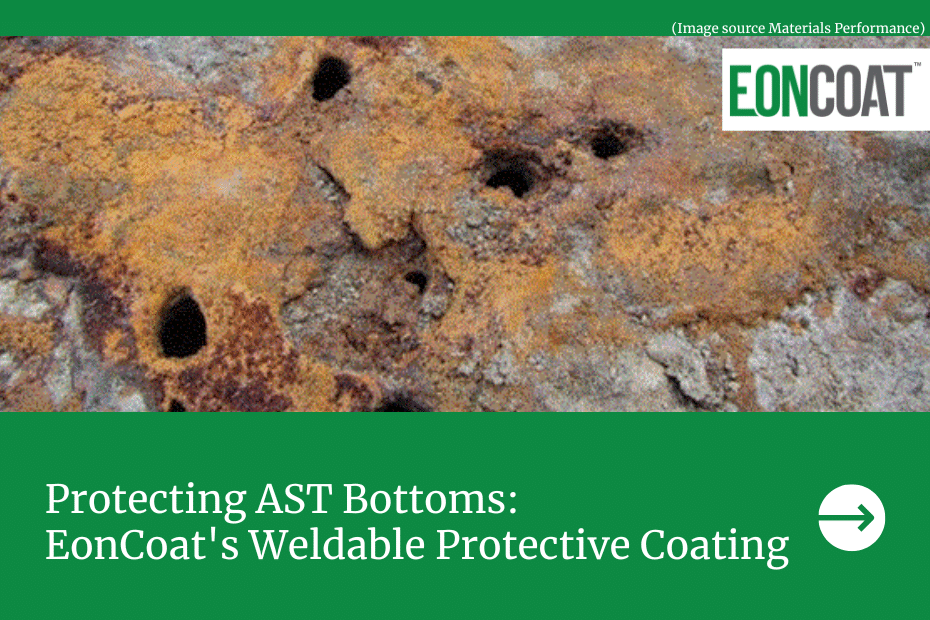
Few destructive forces have plagued humanity with such determination and vigor as corrosion.
The word corrosion is derived from the Latin term “corrodere”, which, when translated literally, means to “gnaw through.” Since the dawn of our modern age, mankind has struggled to find ways to fight against this process. But through experimentation, and ground-breaking feats of engineering, scientists have determined what causes corrosive elements to react to surfaces. They have also developed chemical agents which can prevent such reactions from occurring at all.
Let’s look back at how corrosion prevention has evolved and the best options for today’s industries.
Antifouling Paint
Evidence of antifouling paint has been discovered in documents, like papyrus scrolls, from as early as 412 B.C. These accounts of antifouling paint are the earliest known point in our history’s timeline of man’s ongoing battle with corrosion. In this particular era, a mixture of arsenic, sulfur, and chain oil was used to combat the effects of corrosion on a ship’s wooden hull. By 1625 A.D., William Beale had perfected the technique — concerning advances at the time — and became the first patent holder of antifouling paint. His formula was a more complex mixture of iron powder, copper, and cement. This protective coating ensured that even when fouling organisms adhered to a boat’s surface, they could be scrubbed away with ease. In a time when ships took years and fortunes to build, anti-corrosion techniques proved a valuable asset to investments.
Cathodic Protection
By 1824, antifouling paints were found to significantly poison water supplies, vegetation, and localized fish and mammal populations. To combat this terrible consequence, copper sheathing was adopted as a more environmentally friendly corrosion-deterrent. Yet new problems arose. With this dilemma in mind and financing from the British Navy, Sir Humphry Davy theorized that adding another material to the equation could be the key to success. With the help of Michael Faraday, a solution was found in the form of iron and zinc. A breakthrough was made by implementing another coat of more cost-effective metals: the theory of electrochemical protection. Corrosive agents targeted the iron and zinc coatings and left the underlying steel/wood hull in near pristine condition. This new approach to preventing corrosion was gentler on the environment and also more effective in achieving its purpose.
Anti-Corrosive Coating
Techniques for corrosion prevention have evolved significantly since Davy and Beale’s time — in fact, they’re almost incomparable. The method has been perfected in recent years by introducing a protective coating for steel. Modern phosphate ceramic steel coatings, like EonCoat, allow for complete corrosion prevention instead of simply delaying the process.
As EonCoat is applied to carbon steel, a magnesium iron phosphate alloy layer forms. This alloy layer is chemically bonded to the steel making it impossible for moisture or oxygen to ever come in contact with the substrate. Above the steel, 20 mils of inhibitor in the form of phosphates and silicates are present to continually re-alloy the steel should someone mechanically damage the coating.
A lot has changed in the millenniums that have come to pass since the initial discovery of corrosive defense techniques. But one thing remains the same: the determination of man to find techniques for making our materials last longer. A metal that can withstand the elements is invaluable to modern industries.
When put in this context, the question isn’t whether or not you should invest in these processes, but why you haven’t done so already.

Ready to Learn More About EonCoat?