You are tired of the costs of corrosion! When you see corrosion on your carbon steel asset, you see the money you would rather spend elsewhere. According to the SSPC, corrosion of metals costs US $276 billion annually.
classifications and types of corrosion.
Corrosion is the process of decay on a material caused by a chemical reaction to its environment. The reaction is typically in the form of oxidation. Corrosion of metal occurs when an exposed surface comes in contact with a gas or liquid, and the process is accelerated by exposure to warm temperatures, acids, and salts. Most metals are susceptible to corrosion, but all materials are subject to degradation. For example, corrosion of the polymers used to insulate coatings on electrical wiring has been a concern in older airplanes.
Carbon steel is the most widely used material for engineering applications, consisting of about 85% of all steel production each year. Even though carbon steel is susceptible to corrosion, it is used in everything from nuclear power and fossil fuel power plants to oil and gas refining and pipelines.
CLASSIFICATIONS OF CORROSION
Corrosion can be classified into three categories based on the environment.
- Atmospheric Corrosion: The types of atmospheres are described as rural, industrial, or marine.
- Aqueous Corrosion: Carbon steel pipes are used to transport water and are often submerged in water. The higher the acidity level of the water, the faster the corrosion rate of attack.
- Soil Corrosion: The rate of soil corrosion on carbon steel varies. Soil with high moisture content, high acidity, high dissolved salts, and high electrical conductivity are the most corrosive. Learn how to defend your steel assets from this type of corrosion with
TYPES OF CORROSION
These are the common varieties of corrosion:
- General Attack Corrosion: The most common form of corrosion, general attack corrosion is created by an electrical or electrochemical reaction that breaks down the surface of exposed metal, causing it to weaken until it fails.
- Localized Corrosion: As the name suggests, localized corrosion attacks one part of the metal. There are three types of localized corrosion:
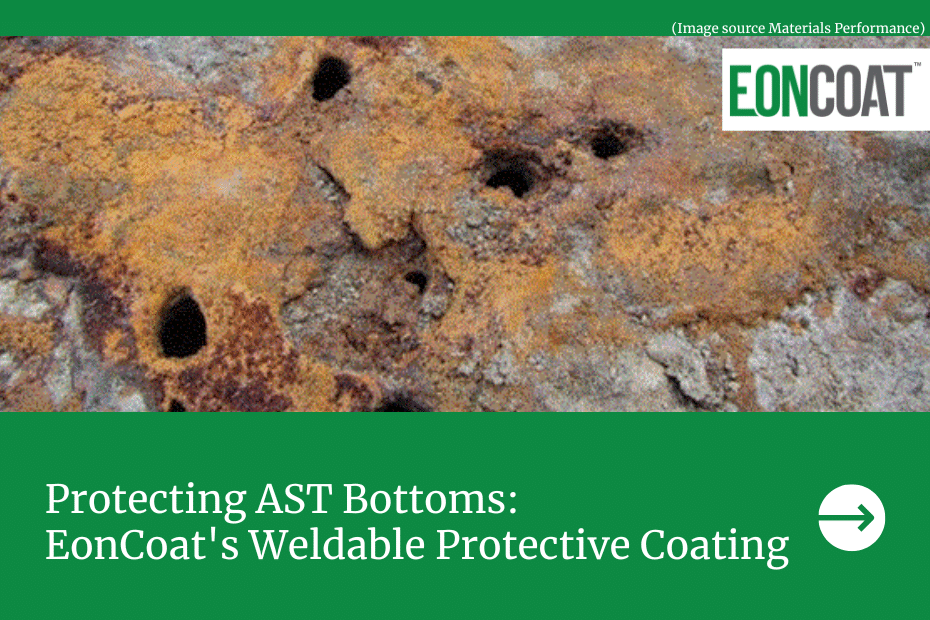
- Pitting is the result of a small hole forming in the metal. The corrosion of this area breaches the metal and leads to failure.
- Crevice corrosion frequently happens in a stagnant microenvironment, much like the space located beneath gaskets and clamps. This type of corrosion can be caused by acidity or oxygen depletion inside a crevice.
-
-
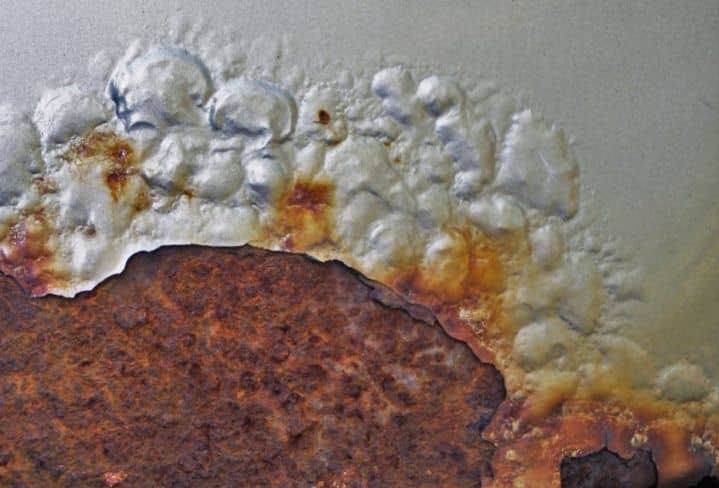
- Filiform corrosion occurs when water breaks through the coating underplated or painted surfaces. This type of corrosion starts at places where there are small flaws in the coating. It eventually spreads and causes weakness in the structure.
-
- Galvanic Corrosion: This type of corrosion happens when two separate metals are placed in a corrosive electrolyte. This creates a galvanic couple between the metals. One metal is now the anode, while the other becomes the cathode. The metal which is the anode now corrodes more quickly than usual, while deterioration slows on the cathode.
- Environmental Cracking: This type of corrosion is the result of a number of conditions in the environment, such as temperature, chemicals, and stress. There are four kinds of environmental cracking:
- Stress Corrosion Cracking (SCC)
- Corrosion fatigue
- Hydrogen-induced cracking
- Liquid metal embrittlement
- Flow-Assisted Corrosion: This occurs when wind or water dissolves a protective oxide layer on the surface of a metal, and then exposes the metal to deterioration.
- Intergranular Corrosion: Intergranular corrosion occurs when the metal contains impurities, resulting in electrochemical or chemical corrosion on the metal’s grain boundaries.
- Dealloying: This type of corrosion is the result of an attack on an alloy’s specific chemical element.
- Fretting Corrosion: This happens where there is repeated surface motion that can come from vibration.
- High-Temperature Corrosion: This is caused by corrosive compounds formed during combustion, and may also be produced by sulfidation, carbonization, and high-temperature oxidation.
PREVENTING CORROSION
There are several techniques that can or slow – or even stop – corrosion:
- Environmental Modification: This is simply changing the environment of the metal to reduce unwanted chemical reactions, such as limiting contact with rain or seawater.
- Metal Selection and Surface Conditions: Understanding the causes of corrosion leads to selecting the right type of metal. Monitoring of surface conditions for such things as cracks and crevices can reduce rates of corrosion.
- Cathodic Protection: This is an electrochemical process that protects against galvanic corrosion (discussed above). Unwanted anodic sites on a metal’s surface are converted to cathodic sites by applying an opposing current.
- Corrosion Inhibitors: These are chemicals that react with the surface of the metal or the gases in the environment that cause corrosion.
- Plating: Metallic plating, such as electroplating, can inhibit corrosion and provide an attractive finish to the surface.
- Coatings: Paints and other organic coatings can protect metals from the corrosive effects of environmental gases. This is the best
THE ADVANTAGES OF COATINGS
There are numerous benefits to using. Here are some of the most common reasons:
- Coatings can be applied onsite, as opposed to galvanizing.
- Coatings can stabilize the surface of the steel, so it will not react to the corrosive factors in the environment.
- Coatings can cover the substrate, leaving no gaps for corrosion to begin forming.
- Coatings are successfully used in the oil and gas industry, including petroleum storage tanks and pipeline exteriors.
- Coatings minimize downtime, which saves money in oil and gas applications.
Regular coatings that sit on top of the carbon steel surface are one thing to which you are accustomed.
However, coatings that combine with the steel to form an alloy are revolutionary. This is EonCoat!
The Safest & Permanent Solution
EonCoat is the newest solution, the best solution – and your permanent solution – to corrosion. We save you money in so many ways.
- EonCoat saves you money by eliminating the frequent reapplication of coatings. Our product gets stronger over time, not weaker. See information about our warranty.
- We have practically eliminated downtime for applications. Our product cures in under 15 minutes.
- EonCoat reduces the risk of illness or injury to your employees who are involved in coating applications. Reduced employee risk always reduces your expenditures.
- We have no VOCs
- No toxins
- No odor
- Are not flammable.
- We are environmentally safe. There are no extraordinary disposal costs for EonCoat.
Reach out to us & start a dialogue about how EonCoat can meet your specific needs!

Ready to Learn More About EonCoat?