Unlike barrier coatings, EonCoat works as a surface treatment that alloys the carbon steel, allowing it to protect itself. Alloying the metal surface prevents rust from developing. Let’s take a closer look at the alloying process.
Definition of an Alloy
An alloy is a metal that’s combined with other substances to create a new metal with superior properties. For example, the alloy may be stronger, harder, tougher, or more malleable than the original metal. Alloys are often thought to be a mixture of two or more metals. However, this is a misconception, as alloys can be composed of one metal and other non-metallic elements.
The predominant metal in the alloy is called the base metal. The other metals or elements added to the alloy are called alloying elements.
Examples of Alloys
In addition to increasing the strength of a metal, alloying may change other properties, including the resistance to heat, corrosion resistance, magnetic properties, or electrical conductivity.
- Steel is created from iron and carbon. Iron is a brittle metal, so it’s not suitable for use as a building material for constructing bridges and buildings. Structures created from iron would eventually collapse. Because it’s tough and has high tensile strength, steel is an ideal construction material.
- Stainless steel, an alloy made from iron and chromium, is more resistant to corrosion and staining when it comes in contact with water as opposed to iron and carbon steel.
- Aluminum is soft and relatively weak. Its strength can be increased by adding other elements, including zinc, copper, magnesium, and manganese. When aluminum contains added elements, it’s known as an aluminum alloy.
The Alloying Process
To create an alloy, the metals (or a metal and a nonmetallic element) are heated until they are molten. The two elements are mixed and the solution is poured into metal or sand molds to solidify. The resulting alloy is a combination of the two elements. Typically, the primary ingredient is melted first, and the others are added to it.
Using Alloying to Prevent Corrosion
We’ve seen that alloys can be created to increase a metal’s resistance to corrosion. The traditional method used to prevent corrosion was to cover the metal with a surface coating, such as a polymer. This creates a barrier between the surface of the metal and the elements.
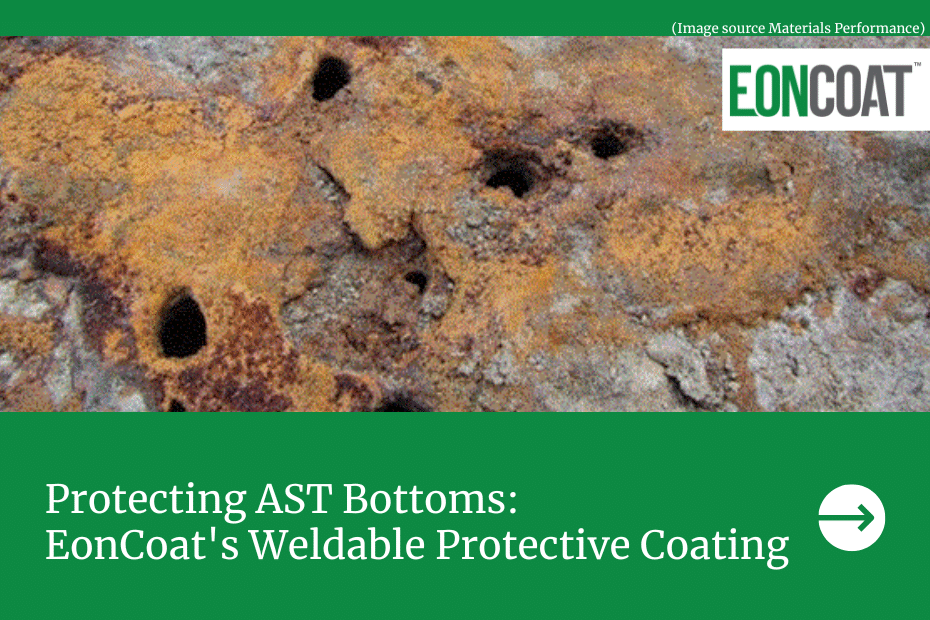
EonCoat isn’t a barrier coating. Fundamentally different from a polymer you paint on the surface of a metal, it’s a surface treatment that actually alloys the steel that it comes into contact with. Since rust starts at the surface of a metal, if the surface is alloyed, there’s nothing exposed, and therefore, no place for rust to form.
How EonCoat Works
EonCoat is sprayed directly onto steel. The acid in the formula reacts with the steel to create an amorphous magnesium iron phosphate layer that’s only 2 microns thick, which is the first line of defense to prevent corrosion. EonCoat’s chemically bonded phosphate ceramic is the second line of defense; the ceramic topcoat continually leaches phosphate to keep rust from forming.
It’s important to keep in mind that EonCoat doesn’t just cover the metal. It actually becomes part of the metal as an alloy, something polymer coatings can’t do. Polymer relies on weaker mechanical bonds and merely sits on top of the metal. Once a polymer coating is scratched, moisture can get in and make contact with the metal. Once this happens, rusting is inevitable. That’s why traditional coatings can only delay the onset of corrosion, while EonCoat actually prevents rust from forming.

Ready to Learn More About EonCoat?